About fine silver
A few weeks ago on Facebook I saw a post about the correct way to anneal fine silver. Being a professional jeweler, I answered with the comment that fine silver does not need annealing as it has no copper or other alloying metals in it and as such, can not work harden.
Well. my opinion was summarily dismissed and the conversation went on with people, mostly hobbyists, either supporting or arguing against my claim. Being the type of personality that I am, I decided to get to the truth. So with several weeks of research, I have found that the truth is somewhere in the middle.
With a few emails, a post on Ganoksin the professional Jewelry Making Forum
Let me start by sharing the information I have found about fine silver as a metal and its properties.
The following information comes from
ASM International (American Society for Metals)
The parts I thought were important to jewelers were on the following pages.
Fine silver (99.9 ag) has the highest electrical and thermal conductivities of all metals. It has a high current capacity, which limits heat generation, and a high thermal capacity, which allows contacts to readily dissipate the heat generated by arcing. …
Metals Handbook 10th edition, Volume 2, Properties and Selection: Nonferrous Alloys and Special-Purpose Materials.Copyright © 1990 by ASM International. page 844
The low boiling and melting points of silver are disadvantages. Fine silver contacts tend to weld easily, and usually have high erosion loss. (see “Silver-Cadmium Alloys” below). The low hardness and low mechanical strength of fine silver result in high rates of mechanical wear.
The following information is from table 4 “Properties of silver metals used for electrical contacts“on page 844. there are 16 rows dealing with special alloys but I will only post those with a silver-copper alloy.
| Alloy | 99.9ag (Fine Silver) | 92.5ag-7.5cu (Sterling Silver) | 90ag-10cu (Coin Silver | 72ag-28cu |
| Solidus Temperature ℃ | 960 | 851 | 775 | 775 |
| Solidus Temperature ℉ | 1760 | 1510 | 1430 | 1430 |
| Electrical conductivity, % IACS | 104 | 88 | 85 | 85 |
| Hardness HR15T Annealed | 30 | 65 | 70 | 79 |
| Hardness HR15T Cold worked | 75 | 81 | 83 | 85 |
| Tensile strength Annealed MPa | 170 | 269 | 276 | 365 |
| Tensile strength Annealed ksi | 25 | 39 | 40 | 53 |
| Tensile strength Cold worked MPa | 310 | 455 | 517 | 552 |
| Tensile strength Cold worked ksi | 45 | 66 | 75 | 80 |
| Density, g/cm3 | 10.51 | 10.34 | 10.31 | 9.95 |
| Elongation in 50mm or 2 in., % Annealed | 55 | 35 | 32 | 20 |
| Elongation in 50mm or 2 in., % Cold worked | 5 | 5 | 4 | 5 |
Additional information from Page 1156-1157
Mass Characteristics
Atomic weight. 107.868
Density. At 20 ℃: 10.49 g/cm3.
Liquid states are belowVolume change on freezing. 5% contraction.
Metals Handbook 10th edition, Volume 2, Properties and Selection: Nonferrous Alloys and Special-Purpose Materials.Copyright © 1990 by ASM International. page 1156-1157
| ℃ | g/cm3 |
| 960.5 | 9.30 |
| 1000 | 9.26 |
| 1092 | 9.20 |
| 1195 | 9.10 |
| 1300 | 9.00 |
Metals Handbook 10th edition, Volume 2, Properties and Selection: Nonferrous Alloys and Special-Purpose Materials.Copyright © 1990 by ASM International. page 1156-1157
Optical Properties
As a result of the high and fairly uniform reflectance in the visible range, silver is considered white, but if human eyes were sensitive to a slightly shorter wavelength region, silver would appear to have color.
the first bit of published information came from a page on Rio Grande, but when I contacted them, they said the information was old and they would look into finding the documentation behind it. They were not able to definitively quote a source[mfn]Rio Grande. “Comparing Silver Hardnesses.” Rio Grande, Comparing Silver Hardnesses, March 1, 2021. https://www.riogrande.com/article?name=Comparing-Silver-Hardnesses-CGl.[/mfn].
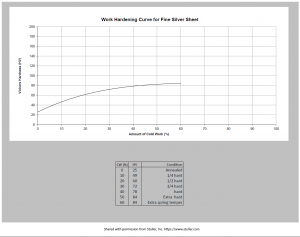
I also had the same experience with Stuller[mfn]”Stuller”, https://www.stuller.com. From conversation on Ganoksin https://orchid.ganoksin.com/t/work-hardening-fine-silver-documentation-sought/59089/10?u=gerald_livings1 [/mfn]. They were able to provide a chart they refer to showing the work hardening of fine silver. This somewhat closely matches the information provided on the Rio Grande website. This information seems to be close to the information in the first section of my post.
Hardening Fine Silver
I want to be clear again that I am talking about fine silver with a purity of 99.9% pure silver. Not Sterling or coin silver, or any kind or silver alloyed with any other metals.
A bit about tempering metal first. As it refers to jewelry alloys, metal temper refers to the hardness and strength that is created in sheet, wire, and other types of metal products by controlled deformation. In jewelers terms, this means cold-working your metal by hammering, drawing through a draw plate, or passing through a rolling mill.
I have not found much documentation about hardening fine silver. I did quite an extensive search online looking for documentable information, and there really is not much. The links at the top of this blog post is pretty much it.
I found several posts talking about using a tumbler to work harden fine silver findings, but I suspect that other than a very shallow surface hardening measured in microns while the interior is still annealed, I do not seeing this as being a workable solution for hardening fine silver findings for jewelry. The most I could find for tumble hardening sterling silver suggests that the hardened surface is only in the neighborhood of 5 microns, most likely less for fine silver. By comparison, a human hair is approximately 70 microns. In my opinion, tumbling fine silver will improve the surface finish of jewelry items, , but will not will not make a significant difference in overall hardness of the fine silver jewelry findings.
One webpage claimed that this could harden fine silver up to 20%, but no documentation was provided. using a tumbler can improve surface The book “Ninth Edition of Metals Handbook, volume 2, titled “Properties and Selection: Nonferrous Alloys and Pure Metals” (American Society for Metals (ASM), 1979) has a chart shows that fine silver can be definitely work hardened. I will post a copy of the chart when I can get a copy from the library. Due to COVID, Inter-library loan is not possible at this time. It suggests that even at a 75% reduction, fine silver will harden no more than 10%. The Stuller chart seems to suggest a increased hardness of 3.4 times so about twice as hard as your fingernail.
Here is what I have found that can be documented for metal hardness form websites I trust to be accurate. Completely annealed fine silver is about 25HV on the vickers hardness scale. As a comparison, the average fingernail is about a 23HV to 31HV[mfn]CORE, https://core.ac.uk/download/pdf/82414794.pdf, CORE’s mission is to aggregate all open access research worldwide and deliver unrestricted access for all.[/mfn].
Hardened fine silver on the chart provided by Stuller puts the hardness at just over 80.
Annealed copper is about 50HV ranging all the way up to about 100HV for hardened pure copper[mfn]MatWeb, Your Source for Materials Information. http://www.matweb.com/search/DataSheet.aspx?MatGUID=9aebe83845c04c1db5126fada6f76f7e[/mfn].
Some Examples of Forging Fine Silver
I would like to include some examples of fine silver that has been forged. Several of my friends make Viking inspired jewelry. To do that, they start with fine silver coins, cut them into sections, and forge them down to the point they can be hammered, drawn, and rolled.
Below are the text from a Facebook post by Shawn Geppert as well as comments with the images he posted. All images are used with permission.

3×2 plait in fine silver 
a triangular money ring in silver 
Cracks from poor annealing.
Finished up a 3×2 plait in fine silver and a triangular money ring in silver last week. The plait is just over 2oz. I started with two 1oz bars and hammered each of the 6 main wires from 1/3oz ingots. The money ring is a 1oz bar that was hammered down to a 4ish gauge rod, then shaped.
Shawn Geppert Facebook post, SCA Metalworking and Jewelers Group (Unofficial Discussion), https://www.facebook.com/groups/865155760229995/permalink/3753524354726440/
This was in reply to a question about how many times he annealed his metal.
absolutely!
Shawn Geppert Facebook post, SCA Metalworking and Jewelers Group (Unofficial Discussion), https://www.facebook.com/groups/865155760229995/permalink/3753524354726440/
I did do annealing during the forging process, as well as the twisting process for the plait. When forging it down the silver work hardens and can flake, which can lead to stress cracks. I try to anneal often to avoid flaking and cracking. When twisting, the wires work harden during twisting so I anneal before starting, during initial twisting at least a couple times, and then before doing the final twist.
Good evening!
Shawn Geppert Facebook post, SCA Metalworking and Jewelers Group (Unofficial Discussion), https://www.facebook.com/groups/865155760229995/permalink/3753524354726440/
I think I have a photo of a piece I was working on that I cracked because it wasn’t annealed well, I can see if I can find that again if it’d be helpful!
Facebook message to gerald Livings, I figured it’d be easier to message you. I found the photo! Here is a bar I was forging down that cracked in a few spots from poor annealing.
Facebook message between Gerald Livings & Shawn Geppert.https://www.facebook.com/messages/t/1294633889
Patrick Thomas Conlin was making a comment in regards to drawing wire on my original post on Facebook. His reply is below.
I can do about 6 pulls on fine silver wire before annealing, so three gauges, half that in sterling. When I pour an ingot, I hammer forge it down by 1/3 before annealing, then go to the mill.
Gerald Livings, https://www.facebook.com/gerald.loosehelm/posts/2255679717898653
So what does this mean?
In my opinion, fine silver can be hardened through controlled deformation. Hammering, drawing, and rolling that significantly deforms the metal and changes the grain structure. But as a jeweler, this is fairly meaningless for what we do except in very narrow circumstances where you are forging your metal.
For the vast majority of jewelers, I do not see the need to anneal fine silver for the very slight difference it will make in your designs. The opposite is true as well. There is no need to work harden your fine silver as the amount of hardening is insignificant for most applications.
If you have opinions, documentation, thoughts and comments, then please comment and I will add to the post so it is as accurate as possible.